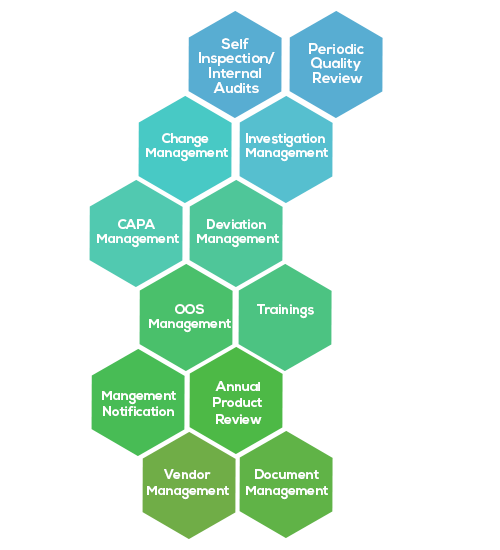
- Designed, created, monitored and reviewed periodically to assess state of control in line with current international standards
- Periodic internal & external consultant audits to assure adherence to the quality system
- Corrective and Preventive measures are planned and implemented to address the identified non-conformities and their effectiveness monitored.
- Vendor/supplier and service providers management including Qualifications and technical agreements
- Ongoing training & education of Personnel
- Committed to maintain best quality standards in Research & Development, Contract services and Manufacturing of Pharmaceutical Products through periodic reviews and Continual Improvement of QMS, and Strive to enhance customer satisfaction
QUALITY MANAGEMENT SYSTEM