- Home
- Company
- What We Do
We are the CDMO partner you need for safe and reliable development and manufacturing of high quality pharmaceutical products to support your product portfolio and ultimately, the well-being of your patients.
With a passionate team of highly cross functional technical experts, with world class facilities and cutting-edge technology, and a broad range of fully-integrated services across our network, we deliver bespoke solutions spanning your complete product lifecycle to secure your supply chain at any scale.
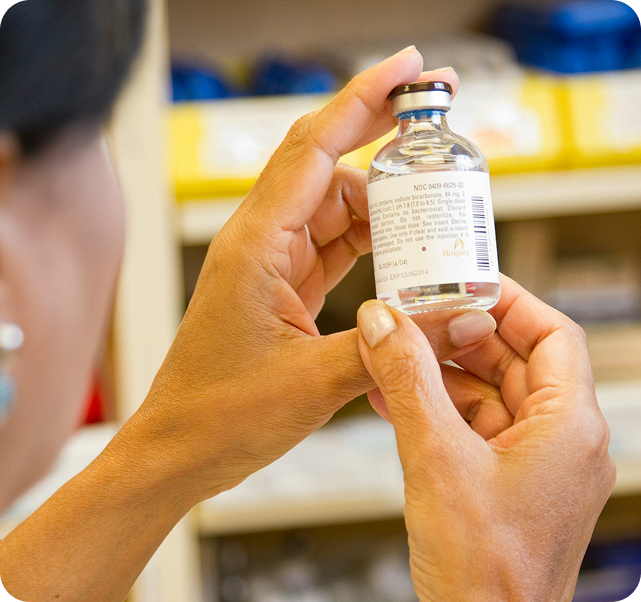
The safe and reliable commercialization of highly potent medicines is crucial to meeting the needs of patients in oncology and other therapeutic indications.
As a global service provider in the production of high potent drug products, we accompany you on your entire product lifecycle from early phase development and the manufacturing of clinical supplies, late-stage registration and validation, to commercial manufacturing and packaging.
Whether it be tablets, capsules, or injectables, we can safely handle them all, with containment capabilities for OELs down to the picogram per cubic meter level.






We are masters in manufacturing of Sterile Injectables by Aseptic processing.
Our team expertises in handling high value Oncology, High potent & Complex injectable products with high degree of sterility assurance.
We have strong track record in sterile injectables backed by many approved ANDA and First to File applications and commercialized drug products in USA & Europe.
We have proven track record in developing multiple complex dosage forms, including sterile liquids and lyophilized vials, prefilled syringes.

We have extensive expertise in managing complex operations like aseptic lyophilization cycle development and optimization. We’ve been doing it for over decades.
There are many complexities facing lyophilized product development
and manufacturing, particularly for sensitive complex product that can destabilize molecules in freeze-dried or frozen formulations. With our extensive experience, we optimize and scale up your lyophilization process to match the needs of your product.

With decades of experience, specialized containment technologies to handle OEL-4 & OEL-5 category products, state-of-the-art facilities, and a proven track record of delivering high-quality OSD products like Tablets, Capsules, powders, we provide the precise infrastructure to support your most demanding OSD requirements at small to large scale manuafacturing of Oncology & High potent products.

Speed matters, but not at the expense of quality. That’s why our experts have developed and optimized robust processes to ensure your product’s integrity.
Our dedicated quality and analytical teams are here to support you as you scale from clinical trials to commercial success.
Discover our world-class CMO/CDMO facility powering high-potent
manufacturing. Empower your operations: Connect with Teyro Labs.
No. 13 (old 29), Thilak street, Parthasarathi Puram,
T Nagar, Chennai-600017, Tamil Nadu, India.